Generic Human Chorionic Gonadotropin Injections (Eutrig-HP 2000iu, 5000iu and 10000iu Injections) – Product Information
Eutrig-HP Injections (Generic Human Chorionic Gonadotrophin Injection) are used for induction of Ovulation in infertility due to anovulation or impaired follicle-ripening. HCG injections are also used for preparation of follicles for puncture in controlled ovarian hyperstimulation programmes (for medically assisted reproductive techniques) and luteal phase support in the female.
In males it is used for Hypogonadotrophic hypogonadism, delayed puberty associated with insufficient gonadotrophic pituitary function and cryptorchidism, not due to anatomical obstruction. You can buy Human Chorionic Gonadotropin injections at the Swiss Pharmacy from only $14 per vial (injection).
We also stock Promifen Tablets (Generic Clomiphene Citrate) which are used for induction of ovulation in women with persistent ovulatory dysfunction who desire pregnancy.
Name of Drug
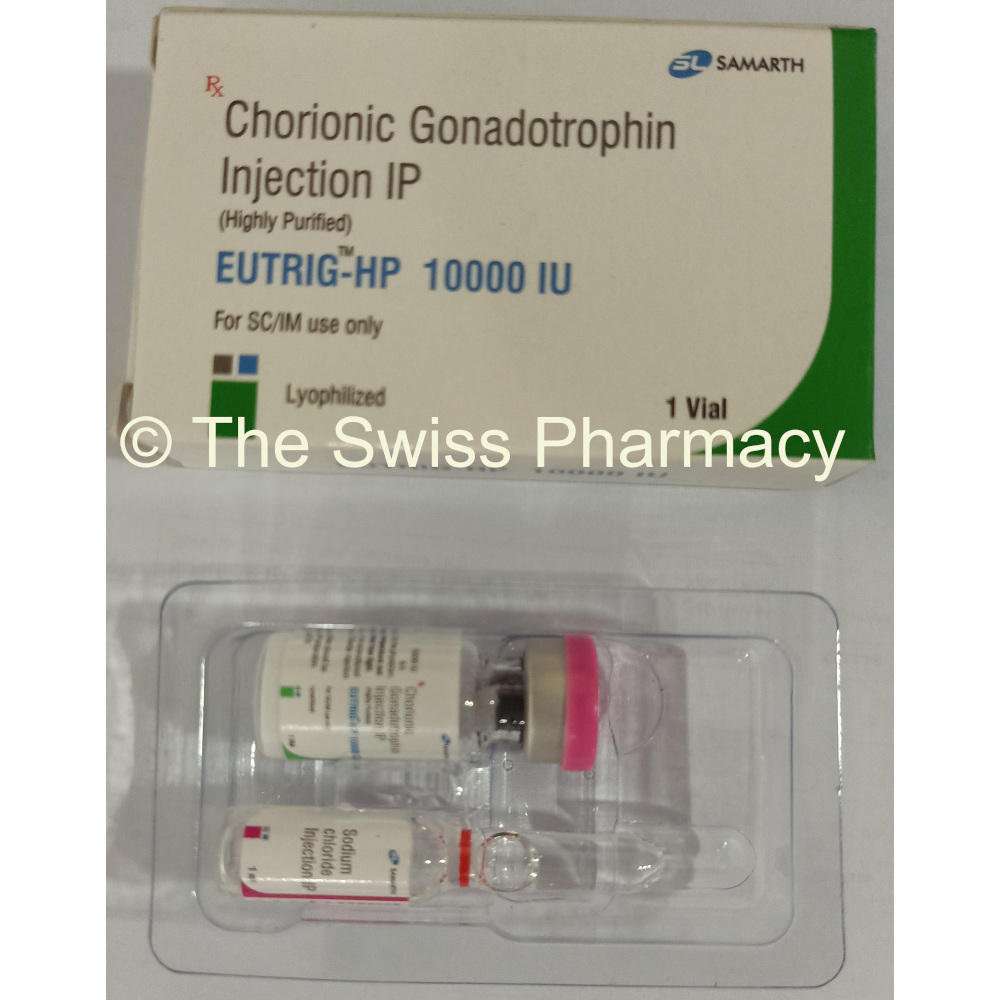
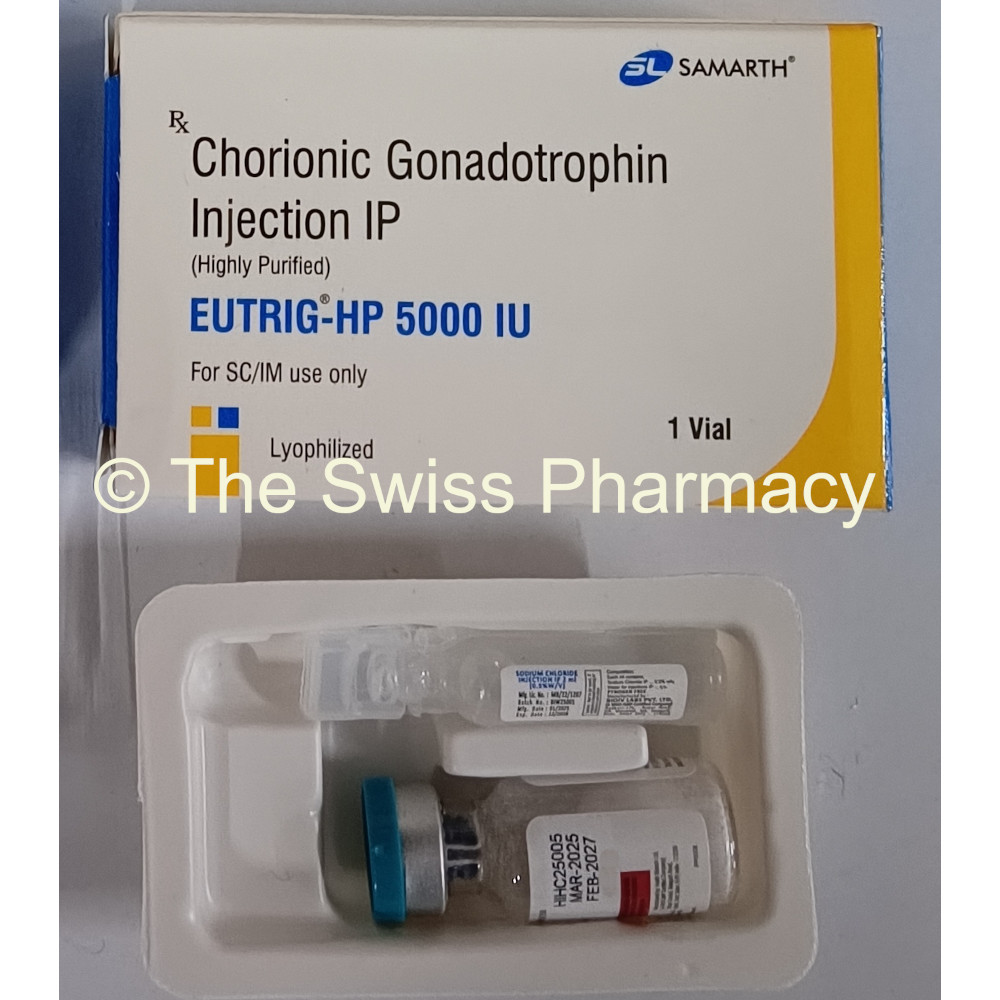
Eutrig-HP 2000iu, 5000iu and 10000iu
Supplied Form for Eutrig-HP 2000iu, 5000iu and 10000iu
Vial (injection)
Packaging : 1 vial + 1 solvent
Manufacturer of Eutrig-HP 2000iu, 5000iu and 10000iu HCG Injections
Samarth Life Sciences Private Limited, India
Website: samarthlife.com
Active Pharmaceutical Ingredient
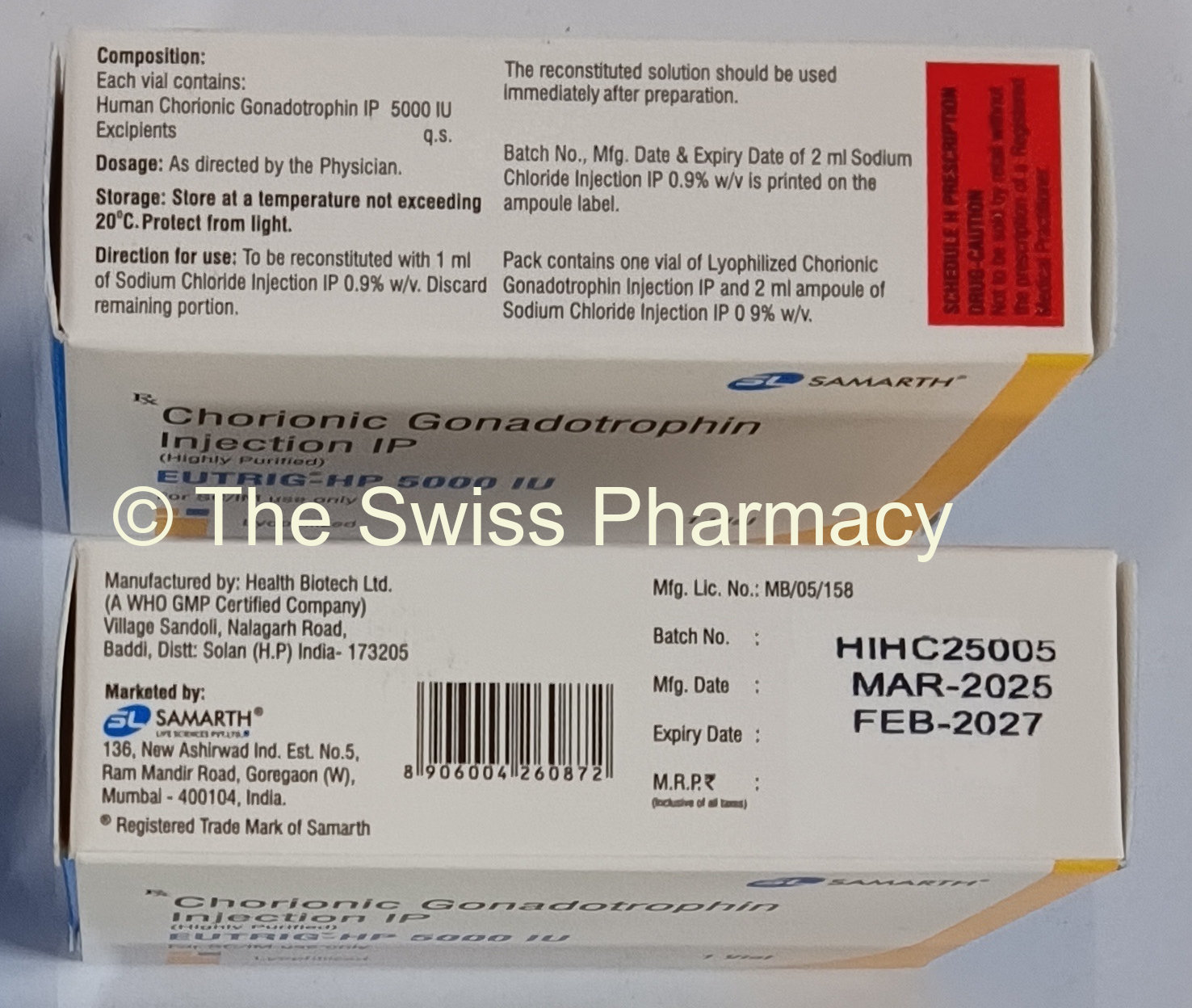
The active ingredient present in Eutrig-HP and Hucog -10000 HP HCG injection is Human Chorionic Gonadotropin. Each vial of Hucog HCG injection contains 5000 iu or 10000 iu of purified Human Chorionic Gonadotropin repectively.
Mechanism of Action
The action of Human Chorionic Gonadotropin is virtually identical to that of pituitary LH, although hCG appears to have a small degree of Follicle Stimulating Hormone (FSH) activity as well.
Chorionic Gonadotropin for Injection, USP is given to males in an effort to stimulate the interstitial cells of the testes (cells of Leydig) to produce androgen. The response to hCG may be considered similar to the effect caused by the interstitial cell stimulating hormone (ICSH) from the anterior lobe of the pituitary. Androgen stimulation in the male leads to the development of secondary sex characteristics and may stimulate testicular descent when no anatomical impediment to descent is present.
Chorionic Gonadotropin for Injection is given in the second phase of the cycle in an effort to maintain the functional integrity of the corpus luteum and to stimulate its secretion of progesterone. Response to hCG may be considered similar to the effect caused by the luteotrophic hormone from the pituitary gland.
Uses of Eutrig-HP 2000iu, 5000iu and 10000iu Injections (Generic Human Chorionic Gonadotrophin Injections)
Eutrig-HP (Human Chorionic Gonadotropin for Injection) is a prescription medicine that contains a hormone to help stimulate healthy ovaries to make eggs. Chorionic Gonadotropin for Injection, USP is used for induction of ovulation and pregnancy in the anovulatory, infertile woman in whom the cause of anovulation is secondary and not due to primary ovarian failure, and who has been appropriately pretreated with FSH-containing preparations. It is usually given in combination with other drugs such as menotropins and urofollitropin. Many women being treated with these drugs usually have already tried Clomiphene alone (e.g., Fertomid Tablets) and have not been able to conceive yet. Eutrig-HP 2000iu, 5000iu and 10000iu Injection is also used in in vitro fertilization (IVF) programs.
In males, LH and chorionic gonadotropin stimulate the testes to produce male hormones such as testosterone. Testosterone causes the enlargement of the penis and testes and the growth of pubic and underarm hair. It also increases the production of sperm. Eutrig-HP is also used to treat Prepubertal cryptorchidism not due to anatomical obstruction. In general, hCG is thought to induce testicular descent in situations when descent would have occurred at puberty.
Eutrig-HP and 10000 HP HCG Injection (Generic Human Chorionic Gonadotropin Injection) – Dosage
The dosage of Eutrig-HP Injection (Generic Human Chorionic Gonadotrophin Injections) for various indications is given below.
Dosage In The Female:
Ovulation Induction In Subfertility Due To Anovulation Or Impaired Follicle-Ripening
Usually, one injection of 5,000 to 10,000 IU Eutrig-HP and 10000 HP to complete treatment with an FS H-c ontaining preparation.
Preparation Of Follicles For Puncture In Controlled Ovarian Hyperstimulation Programs
Usually, one injection of 5,000 to 10,000 IU Eutrig-HP and 10000 HP to complete treatment with an FS H-c ontaining preparation.
Luteal Phase Support:
Two to three repeat injections of 1,000 to 3,000 IU each may be given within nine days following ovulation or embryo transfer (for example, on day 3, 6 and 9 after ovulation induction).
Dosage In The Male:
Hypogonadotrophic Hypogonadism:
1,000-2,000 IU Human Chorionic Gonadotropin, two to three times per week. If the main complaint is subfertility, Human Chorionic Gonadotropin may be given with an additional follitropin (FSH)-containing preparation two to three times per week. This treatment should be continued for at least three months before any improvement in spermatogenesis can be expected. During this treatment testosterone replacement therapy should be suspended. Once achieved, the improvement may sometimes be maintained by hCG alone.
Delayed Puberty Associated With Insufficient Gonadotrophic Pituitary Function:
1,500 IU two to three times a week for at least six months.
Cryptorchidism Not Due To Anatomical Destruction:
Under 2 years of age: 250 IU twice weekly for six weeks.
Under 6 years of age: 500 to 1,000 IU twice weekly for six weeks.
Over 6 years of age: 1,500 IU twice weekly for six weeks.
If necessary, this treatment can be repeated.
Please consult your doctor for exact dosing instructions. Eutrig-HP 2000iu, 5000iu and 10000iu Human Chorionic Gonadotropin Injection should be administered in the dose prescribed by the doctor and for the duration recommended by the doctor.
Missed Dosage Instructions for Generic Human Chorionic Gonadotropin Injections (Eutrig-HP HCG Injections)
For men or boys: If you are giving your own injections, and miss a dose, take it as soon as you remember. If you forget until the next day, skip the missed dose and continue with your schedule. Do not use double or extra doses. Call your doctor if you have any questions.
For women receiving fertility treatment: It is important not to miss a dose, as the success of your fertility treatment depends on proper use of this medication. Call your doctor or health care professional if you are unable to keep an appointment. If you are giving your own injections, do not use double or extra doses.
Eutrig-HP Injections – Contraindications
Eutrig-HP 2000iu, 5000iu and 10000iu Injections are contraindicated in persons with a hypersensitivity (allergy) to Human Chorionic Gonadotropin or any of the other ingredients of this medicine.
Other contraindications include Precocious puberty, prostatic carcinoma or other androgen-dependent neoplasm, prior allergic reaction to HCG. HCG may cause fetal harm when administered to a pregnant woman. Combined HCG/PMS (pregnant mare’s serum) therapy has been noted to induce high incidences of external congenital anomalies in the offspring of mice, in a dose-dependent manner.
Storage Instructions for Eutrig-HP 2000iu, 5000iu and 10000iu Injections
Store Eutrig-HP 2000iu, 5000iu and 10000iu Injection(Generic Human Chorionic Gonadotropin Injections) at controlled room temperature (59°F to 86°F, 15°C to 30°C). When reconstituted, the solution should be kept refrigerated (2°C to 8°C) and should be used within 30 days. Protect from light.
Warnings and Precautions to be taken when using Eutrig-HP 2000iu, 5000iu and 10000iu Injection(Generic Human Chorionic Gonadotropin Injections)
Before using Eutrig-HP 2000iu, 5000iu and 10000iu Injections please inform your doctor about all the medicines that you take including no prescription medications, over the counter medicines and herbal remedies. Tell your doctor about all of your medical conditions, including if you have heart or kidney disease, epilepsy, migraine, or asthma. Also inform your doctor if are pregnant or breastfeeding. Chorionic gonadotropin may cause harm to an unborn baby when given to a pregnant woman. It is not known whether chorionic gonadotropin is excreted in human milk.
Eutrig-HP Injections (Human Chorionic Gonadotropin Injections) should be used in conjunction with human menopausal gonadotropins only by doctors experienced with infertility problems who are familiar with the criteria for patient selection, contraindications, warnings, precautions, and adverse reactions.
In women undergoing ovulation induction, an excessive ovarian response to follicular stimulating agents may lead to the development of ovarian hyperstimulation syndrome if Human Chorionic Gonadotropin is given to induce ovulation or to support the corpus luteum. It is of primary importance that u-hCG should be withheld in such cycles.
Rupture of ovarian cysts with resultant haemoperitoneum
Thromboembolic complications: Thromboembolic events have been reported following gonadotropin/ Human Chorionic Gonadotropin therapy both in association with and separated from OHSS. These included thrombophlebitis, pulmonary embolism, stroke, and arterial occlusion resulting in loss of a limb. In rare cases, thromboembolic events have resulted in death.
Multiple pregnancy: The incidence of multiple pregnancies and births is increased following gonadotropins/u-hCG therapy stimulation and ovulation induction in patients attempting in vivo conception. The risk of multiple pregnancy following ART is related to the number of oocytes/embryos replaced.
Pregnancy testing A false positive result-xnjght be obtained if the test is carried out in a patient who has recently undergone (over the last 7 days) or is still having u-hCG administration.
Infertile women undergoing Assisted Reproductive Technologies (ART) have an increased incidence of ectopic pregnancy.
Rates of pregnancy loss in women undergoing ART are higher than in the normal population.
The incidence of congenital malformations after Assisted Reproductive Technologies (ART) may be slightly higher than after spontaneous conceptions. This slightly higher incidence is thought to be related to differences in parenteral characteristics (e.g. maternal age, sperm characteristics) and to the higher incidence of multiple gestations after ART.
Androgens may cause fluid retention in the male if high doses of Eutrig-HP and 10000 HP HCG Injection are administered. In such cases dosage should be considerably reduced particularly in patients with cardiac or renal disease, epilepsy, migraine or asthma.
Sexual precocity: HCG may cause sexual precocity when administered in young patients for cryptorchidism. If signs are observed, treatment should be stopped. If continued therapy is considered necessary, a reduced dosage regimen should be instituted.
Finally, HCG (Eutrig-HP 2000iu, 5000iu and 10000iu) may induce gynecomastia.
Chance Of Having Ovarian Hyperstimulation Syndrome (OHSS)
Treatment with gonadotropic hormones like Eutrig-HP 2000iu, 5000iu and 10000iu may cause ovarian hyperstimulation syndrome (OHSS). This is a serious medical condition where the ovaries are overly stimulated and the growing follicles become larger than normal. In rare cases, severe OHSS may be life-threatening. Therefore, close supervision by your doctor is very important. To check the effects of treatment, your doctor will do ultrasound scans of your ovaries.Your doctor may also check blood hormone levels.
OHSS causes fluid to build up suddenly in your stomach and chest areas and can cause blood clots to form. Call your doctor right away if you have:
severe abdominal swelling and pain in the stomach area (abdomen)
feeling sick (nausea)
vomiting
sudden weight gain due to fluid build-up
diarrhea
decreased urine output
trouble breathing
Effects On Ability To Drive And Use Machines
As far as known Eutrig-HP 2000iu, 5000iu and 10000iu Injection (Generic Human Chorionic Gonadotropin Injections) has no influence on alertness and concentration
Side Effects of Eutrig-HP 2000iu, 5000iu and 10000iu Injections(Generic Human Chorionic Gonadotropin Injections)
The possible Side Effects of Eutrig-HP 2000iu, 5000iu and 10000iu HCG Injections(Generic Human Chorionic Gonadotropin Injections) are given below:
Immune system disorders: In rare cases generalized rash or fever may occur.
General disorders and administrative site conditions: Eutrig-HP may cause reactions at the site of injection, such as bruising, pain, redness, swelling and itching, have been reported with the use of urinary gonadotrophin preparations. Anaphylaxis and other allergic reactions have occurred with urinary-derived human chorionic gonadotropin (hCG) products. Occasionally allergic reactions have been reported, mostly manifesting as pain and/or rash at the injection site.
In The Female
- Vascular disorders: In rare instances, thromboembolism has been associated with FSH/hCG therapy, usually associated with severe OHSS. Respiratory, thoracic and mediastinal disorders Hydrothorax, as a complication of severe OHSS.
- Gastrointestinal disorders: Abdominal pain and gastrointestinal symptoms such as nausea and diarrhoea, related to mild OHSS. Call your doctor or get medical help right away if you have: severe abdominal or pelvic pain, nausea, vomiting, diarrhea, sudden weight gain, trouble breathing, or decreased or no urination.
- Ascites, as a complication of severe OHSS.
- Reproductive system and breast disorders: Unwanted ovarian hyperstimulation, mild or severe ovarian hyperstimulation syndrome (OHSS).Chorionic Gonadotropin can sometimes stimulate the ovaries too much. This is called ovarian hyperstimulation syndrome (OHSS) and can be a serious medical problem. OHSS may cause pelvic pain or breathing problems, or it may make you urinate less. In rare cases, patients with this problem have had serious lung problems.
- Painful breasts, mild to moderate enlargement of ovaries and ovarian cysts related to mild OHSS.
- Large ovarian cysts (prone to rupture), usually associated with severe OHSS.
- Investigation: Weight gain as a characteristic of severe OHSS.
- Multiple babies: Chorionic Gonadotropin may cause you to be pregnant with twins or more than two babies at the same time.
In The Male
- Metabolism and nutrition disorders
- Water and sodium retention is occasionally seen after administration of high dosages; this is regarded as a result of excessive androgen production.
- Reproductive system and breast disorders
- HCG treatment may sporadically cause gynaecomastia
Generic Human Chorionic Gonadotrophin Injections Overdosage
Seek emergency medical attention if you think you have used too much of Eutrig-HP 2000iu, 5000iu and 10000iu injections (Generic Human Chorionic Gonadotrophin Injections). An overdose of HCG is not expected to produce life-threatening symptoms. The acute toxicity of urinary gonadotrophin preparations has been shown to be very low. Nevertheless, there is a possibility that too high a dosage of hCG may lead to ovarian hyperstimulation syndrome.
Stop using Eutrig-HP and 10000 HP HCG injections and get emergency medical help if you have any of these signs of an allergic reaction: hives; difficulty breathing; swelling of your face, lips, tongue, or throat. Call your doctor at once if you have any of these signs of a blood clot: pain, warmth, redness, numbness, or tingling in your arm or leg; confusion, extreme dizziness, or severe headache.
Common Side effects of Eutrig-HP and 10000 HP (Human Chorionic Gonadotropin Injection) are Headache, irritability, restlessness, depression, fatigue, edema, precocious puberty, gynecomastia, pain at the site of injection.
Hypersensitivity reactions, both localized and systemic in nature, have also been reported.
Generic Human Chorionic Gonadotrophin Injections During Pregnancy
Human Chorionic Gonadotropin Injection has been classified by the US FDA as Pregnancy Category X.
Chorionic gonadotropin may cause fetal harm when administered to a pregnant woman. Defects of forelimbs and central nervous system and alterations in sex ratio have been reported in mice receiving combined gonadotropin and chorionic gonadotropin therapy in dosages to induce superovulation.
Multiple ovulations with resulting plural gestations (mostly twins) have been reported to occur in approximately 20% of pregnancies when conception has followed chorionic gonadotropin therapy.
Eutrig-HP and 10000 HP HCG injections are not indicated during breastfeeding. It is not known whether Human Chorionic Gonadotropin is excreted in human milk.
Buy Eutrig-HP 2000iu, 5000iu and 10000iu Injections Online from Only $14 per Vial
You can buy Generic Human Chorionic Gonadotropin 2000iu, 5000iu and 10000iu injections online at a cheap price from the Swiss Pharmacy. Eutrig-HP 2000iu Injections manufactured by Samarth Life Sciences Private Limited, India are priced at only $14 per unit if you place an order for 20 vials (injections), whereas Eutrig-HP 5000iu Injections are available for $18 per unit.