Nolvadol (Generic Tamoxifen Citrate Tablets) – Product Information
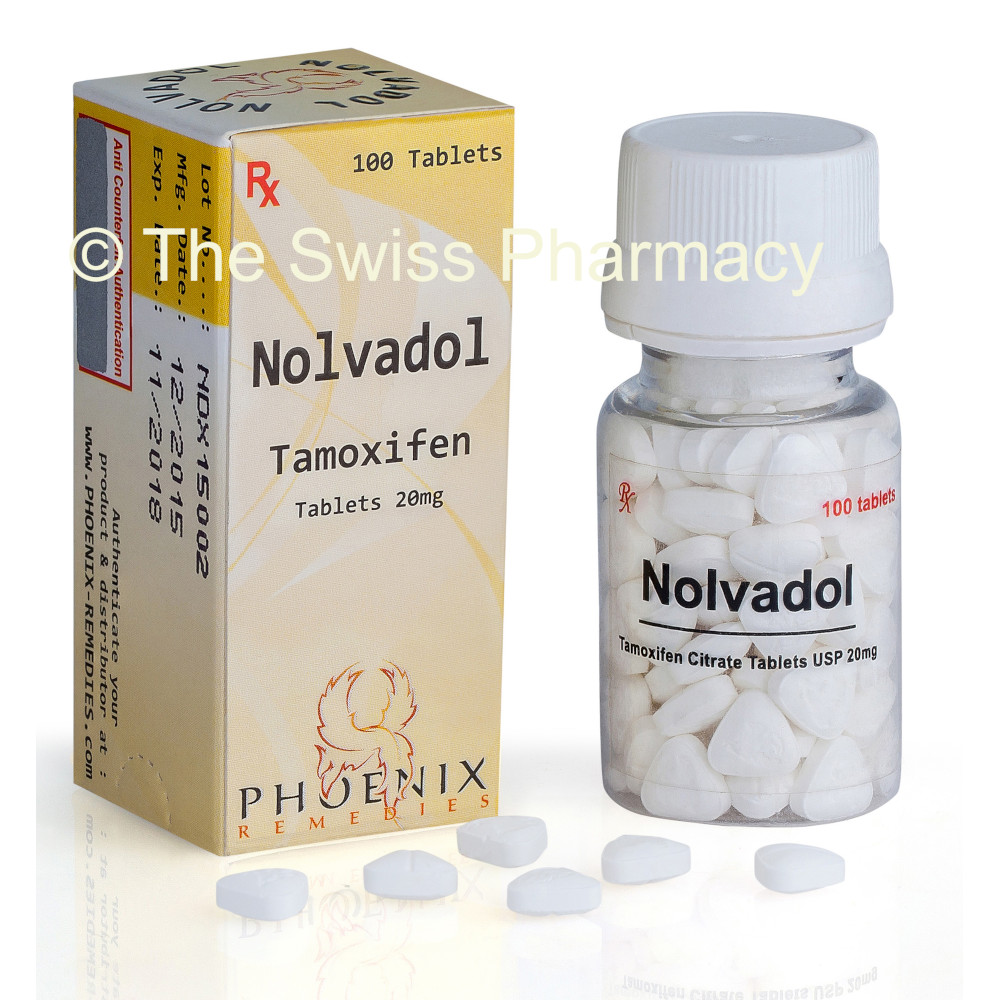
Nolvadol (Generic Tamoxifen Citrate Tablets) a nonsteroidal antiestrogen, is used for the treatment of breast cancer and the treatment of anovulatory infertility. Nolvadol tablets, manufactured by Phoenix Remedies are available for only $0.32 a Pill.
We also have in stock Generic Exemestane tablets (Aromex Tablets) which is used for the adjuvant treatment of postmenopausal women with estrogen-receptor positive early breast cancer who have received two to three years of Tamoxifen Citrate.
Name of Drug
Nolvadol Tablets
Manufacturer of Nolvadol Tablets (Tamoxifen Citrate Tablets)
Phoenix Remedies
Website: phoenix-remedies.com
Active Pharmaceutical Ingredient
The active pharmaceutical ingredients contained in Nolvadol tablets Tamoxifen Citrate. Each Nolvadol tablet contains 30.4 mg Tamoxifen Citrate, an amount equivalent to 20 mg of Tamoxifen.
Mechanism of Action
Nolvadol tablets contain the active ingredient Tamoxifen Citrate which works by blocking the actions of oestrogen, a female sex hormone. It belongs to a group of medicines known as non-steroidal anti-oestrogens.
Uses of Nolvadol (Tamoxifen Citrate tablets)
Metastatic Breast Cancer: Generic Tamoxifen Citrate tablets, are effective in the treatment of metastatic breast cancer in women and men.
Adjuvant Treatment of Breast Cancer: Generic Tamoxifen tablets, are used for the treatment of node-positive breast cancer in women following total mastectomy or segmental mastectomy, axillary dissection, and breast irradiation.
Ductal Carcinoma in Situ (DCIS): Generic Tamoxifen tablets are indicated to reduce the risk of invasive breast cancer in women with DCIS, following breast surgery and radiation.
Reduction in Breast Cancer Incidence in High Risk Women: Generic Tamoxifen Citrate tablets are indicated to reduce the incidence of breast cancer in women at high risk for breast cancer. Nolvadol (Tamoxifen Citrate Tablets) is indicated only for high-risk women. "High risk" is defined as women at least 35 years of age with a 5-year predicted risk of breast cancer (greater than 1.67%), as calculated by the Gail Model.
Anovulatory Infertility: For the treatment of anovulatory infertility Nolvadol (Tamoxifen Citrate tablets) may be a better choice in some patients who fail to either ovulate or conceive with Clomiphene due to its favorable effect on the cervical mucus and endometrium.
Anovulatory Infertility: For the treatment of anovulatory infertility Nolvadol (Tamoxifen Citrate tablets) may be a better choice in some patients who fail to either ovulate or conceive with Clomiphene due to its favorable effect on the cervical mucus and endometrium.
Male Infertility:Tamoxifen Citrate improves fertility in males with infertility by disinhibiting the hypothalamic-pituitary-adrenal axis and thereby increasing the secretion of luteinizing hormone and follicle-stimulating hormone and increasing testicular testosterone production.
Gynecomastia: Generic Tamoxifen is used to prevent or treat gynecomastia. This medicine is taken as a preventative measure in small doses, or used at the onset of any symptoms such as nipple soreness or sensitivity. Other medicines are administered for similar purposes such as Clomiphene Citrate and the anti-aromatase drugs which are used in order to try to avoid the hormone-related adverse effects.
Nolvadol (Tamoxifen Citrate tablets) – Dosage
Swallow Nolvadol tablets with a glass of water at about the same time each day. Do not chew or crush the tablets. Nolvadol tablets can be taken with or without food. The dosage of this medicine for various indications is given below:
Breast cancer: The dosage range for adults (including the elderly) is 20 to 40 mg daily given either in divided doses twice daily or as a single dose once daily. In early disease, it is currently recommended that treatment is given for not less than 5 years. The optimal duration of tamoxifen therapy remains to be determined. Dosages greater than 20 mg per day should be given in divided doses (morning and evening).
Ductal Carcinoma in Situ (DCIS): The recommended dose is Nolvadol (Tamoxifen Citrate tablets) 20 mg daily for 5 years.
Reduction in Breast Cancer Incidence in High Risk Women: The recommended dose is Nolvadol tablets 20 mg daily for 5 years. There are no data to support the use of Tamoxifen Citrate other than for 5 years.
Anovulatory Infertility: Before starting any course of treatment, whether initial or subsequent, the possibility of pregnancy must be excluded. In women who are menstruating regularly, but with anovular cycles, the initial course of treatment consists of 20 mg given daily on the second, third, fourth and fifth days of the menstrual cycle.
Missed Dosage Instructions for Nolvadol Tablets
If it is almost time for your next dose, skip the dose you missed and take your next dose of Nolvadol at your scheduled time. Otherwise, take the missed dose as soon as you remember, and then go back to taking your tablets as you would normally.
Do not take a double dose to make up for the dose you missed.
Nolvadol Tablets – Contraindications
Nolvadol tablets are contraindicated in patients with a hypersensitivity to Tamoxifen Citrate or any ingredient present in Nolvadol tablets. Nolvadol tablets are also contraindicated in the following cases:
Reduction in Breast Cancer Incidence in High Risk Women and Women with DCIS: Nolvadol (Tamoxifen Citrate tablets) is contraindicated in women who require concomitant coumarin-type anticoagulant therapy or in women with a history of deep vein thrombosis or pulmonary embolus.
Pregnancy: Pre-menopausal patients must be carefully examined before treatment for breast cancer or infertility to exclude the possibility of pregnancy.
Concurrent Anastrozole therapy: Concurrent use of Anastrozole and Tamoxifen (Nolvadol tablets) may result in reduced anastrozole plasma levels.
Treatment for infertility: Patients with a personal or family history of confirmed idiopathic venous thromboembolic events or a known genetic defect.
Storage Instructions for Nolvadol Tablets
Store Nolvadol tablets at controlled room temperature 20°C to 25°C. Protect from light. Avoid excessive heat (over 104°F/40°C). DO NOT freeze or refrigerate. Store in original packaging in order to protect from light. Use within 3 months of opening.
Warnings and Precautions to be taken when using Nolvadol (Tamoxifen Citrate tablets)
Before using Nolvadol tablets please inform your doctor about all the medicines that you take including no prescription medications, over the counter medicines and herbal remedies.
Effects in Metastatic Breast Cancer Patients: As with other additive hormonal therapy (estrogens and androgens), hypercalcemia has been reported in some breast cancer patients with bone metastases within a few weeks of starting treatment with Tamoxifen. If hypercalcemia does occur, appropriate measures should be taken and, if severe, Tamoxifen should be discontinued.
Effects on the Uterus-Endometrial Cancer and Uterine Sarcoma: An increased incidence of endometrial changes including hyperplasia, polyps, cancer and uterine sarcoma (mostly malignant mixed Mullerian tumours) has been reported in association with Tamoxifen treatment.
Non-Malignant Effects on the Uterus: An increased incidence of endometrial changes including hyperplasia and polyps have been reported in association with Tamoxifen treatment.
Thromboembolic Effects of Tamoxifen: There is evidence of an increased incidence of thromboembolic events, including deep vein thrombosis and pulmonary embolism, during Tamoxifen therapy.
Effects on the liver: Liver cancer: In the Swedish trial using adjuvant tamoxifen 40 mg/day for 2 years to 5 years, 3 cases of liver cancer have been reported in the Tamoxifen-treated group vs. 1 case in the observation group.
Effects on the liver: Non-malignant effects: Tamoxifen has been associated with changes in liver enzyme levels, and on rare occasions, a spectrum of more severe liver abnormalities including fatty liver, cholestasis, hepatitis and hepatic necrosis. A few of these serious cases included fatalities.
Other cancers: A number of second primary tumors, occurring at sites other than the endometrium, have been reported following the treatment of breast cancer with Tamoxifen in clinical trials.
Effects on the Eye: Cases of visual disturbances, including corneal changes, decrement in color vision perception, retinal vein thrombosis, and common reports of retinopathy have been reported in patients receiving Tamoxifen Citrate tablets. Cataracts and the need for cataract surgery have been reported in association with the administration of Tamoxifen Citrate.
Leucopenia: Tamoxifen should be used cautiously in patients with existing leucopenia or thrombocytopenia. Leucopenia has been observed following the administration of Tamoxifen sometimes in association with anaemia and/or thrombocytopenia.
Tamoxifen Citrate (Nolvadol Tablets) Side Effects
The most common Tamoxifen Citrate (Nolvadol Tablets) side effect in patients treated with for metastatic breast cancer, is hot flashes.
Other Nolvadol side effects which are seen infrequently are hypercalcemia, peripheral edema, distaste for food, pruritus vulvae, depression, dizziness, light-headedness, headache, hair thinning and/or partial hair loss, and vaginal dryness.
During trials for the indication reduction in breast cancer incidence in high risk women side effects reported include Hot Flashes, Vaginal Discharges, Vaginal Bleeding, Platelets decreased. Other Toxicities reported include Mood, Infection/Sepsis, Constipation, Alopecia, Skin and Allergy.
Nolvadol (Tamoxifen Citrate tablets) – Drug Interactions
The drug Interactions of are given below:
When Tamoxifen Citrate tablets are used in combination with coumarin type anticoagulants, a significant increase in anticoagulant effect may occur. Where such co-administration is started, careful monitoring of the patient is recommended.
When Tamoxifen Citrate is administered in combination with cytotoxic agents, there is increased risk of thromboembolic events occurring.
The use of Tamoxifen Citrate in combination with an aromatase inhibitor as adjuvant therapy has not shown improved efficacy compared with Tamoxifen alone.
The known principal pathway for tamoxifen metabolism in humans is demethylation, catalysed by CYP3A4 enzymes. Pharmacokinetic interaction with the CYP3A4 inducing agent Rifampicin, showing a reduction in tamoxifen plasma levels has been reported.
Cytochrome P450 2D6 (CYP2D6) plays an important role in the metabolism of Tamoxifen Citrate. CYP2D6 helps convert Tamoxifen Citrate to Endoxifen (a potent active metabolite of Tamoxifen). Therefore, co-administration of Tamoxifen with CYP2D6 inhibitors such as Paroxetine, Fluoxetine and Bupropion may reduce plasma levels of Endoxifen and should be avoided where possible.
Buy Generic Tamoxifen 20 mg Tablets Online at Only $0.32 per Pill
You can buy Generic Tamoxifen 20 mg tablets online at a cheap rate from the Swiss Pharmacy. Nolvadol pills are priced at only $0.32 per unit if you place an order for 1000 tablets.